The Coral Bleaching Debate:
Is Bleaching the Legacy of a
Marvelous Adaptation Mechanism
or A Prelude to Extirpation?
A Warm Evolutionary Legacy
Despite
increasing confirmation of the Adaptive Bleaching Hypothesis and its
ability to explain coral resilience, most people are unaware of its
debate within the scientific community. The ability to rapidly adjust to
changing environments by modifying their symbiotic partnerships has
been the key to their success for millions of years. As one expert wrote,
the “flexibility in coral–algal symbiosis is likely to be a principal
factor underlying the evolutionary success of these organisms”.
Our
modern day reef-building corals first evolved in exceedingly warm and
stable climates when deep ocean temperatures were 10°C higher than today
and palm trees dotted the Antarctic coast. As ice caps began to form in
Antarctica ~35 million years ago sea levels fell and warm
epi-continental seas dried. After ocean depths had cooled
for another 30 million years, Arctic ice caps began to form and the
earth entered an age with multiple episodes of glacier advances and
retreats causing sea levels to rise and fall. Just eighteen thousand
years ago during the last glacial maximum, all our shallow reefs did not
exist, as sea levels were 400 feet lower than today.
The 35 million year cooling trend increasingly restricted reef-building corals
to more tropical latitudes where winter water temperatures remain above
16 to 18 °C. As their evolutionary history would predict, today’s
greatest concentrations and greatest diversity of corals are found in
the earth’s persistently warmer waters, like the Indo-Pacific Warm Pool.
Likewise species inhabiting our warmest waters have undergone the fewest episodes of severe coral bleaching.
Given their evolutionary history, coral’s greatest achievement has been
enduring bouts of sustained climate cooling and rapid temperature
swings. Even during warm interglacials coral battled cold temperatures
dips. Studies of 7000-year-old fossil coral reefs in the South China Sea
revealed high coral mortality every 50 years due to winter cooling
events. Indeed most researchers believe past coral extinctions were most
commonly due to cold events. Accordingly research
has estimated that during the cold nadir of each ice age, coral reef
extent was reduced by 80% and carbonate production was reduced by 73%
relative to today.
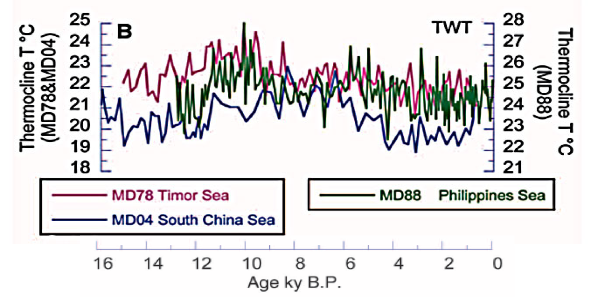
As the last ice age ended, coral expanded
their range with warming temperatures. At the peak of the Holocene
Optimum 10,000 years BP (Before Present), coral adapted to tropical
ocean temperatures in the heart of the Coral Triangle that were 2.1 °C warmer
than today. As illustrated above, temperatures cooled since then but
frequently spiked or plummeted by 2 to 3 degrees over the course of a
few centuries. One thousand years ago during the Medieval Warm Period,
coral thrived in Pacific water masses that were ~0.65° warmer than in
recent decades, then cooled ~0.9°C by the 1700s. Given coral’s
evolutionary history, it is unlikely coral were better adapted to 1800s
Little Ice Age temperatures versus Medieval Warm Period or 20th century
temperatures. Emerging research now suggests coral bleaching has been
an integral part of corals’ adjustment mechanisms to an ever-changing
environment.
Coral Mortality and Resilience
There are 4 widespread misconceptions about bleaching propagated by tabloid media hyping climate doom and researchers like Hoegh-Guldberg. To clarify:
1 Bleaching is not always driven by warming temperatures
2 Bleaching is not responsible for most coral mortality.
3 Coral can rapidly respond to disturbances and replace lost cover within a decade or less.
4
Bleaching, whether or not it results in coral mortality, is part of a
natural selection process from which better-adapted populations emerge.
1. Multiple Causes of Bleaching
In
contrast to researchers like Hoegh-Guldberg who emphasizes coral
bleaching as a deadly product of global warming, bleaching is a visible
stage in a complex set of acclimation mechanisms during which coral
expel, shift and shuffle their symbionts, seeking the most beneficial
partnership possible. Bleaching can be induced by stressful interactions
between temperatures, disease, heavy rains, high irradiance from clear
skies and competition with seaweeds. Indeed abrupt warm water events
like El Nino have induced widespread bleaching and high mortality. But cold winters or La Nina induced upwelling of colder waters have also induced bleaching.
NOAA has also contributed to these misconceptions by overemphasizing just warm-event bleaching. On NOAA‘s web page
“What is Coral Bleaching”, NOAA reported, “the U.S. lost half of its
coral reefs in the Caribbean” in one year due to warmer waters. But the
Caribbean’s main cause of lost reefs was due to an outbreak of the White
Band disease in 1981-82. White band specifically targets members of the
genus Acropora, like the Staghorn and Elkhorn coral, reducing by 80% of their cover that once dominated the Caribbean reefs. However since the mid 80s experts reported coral cover has changed relatively little.
NOAA
also downplayed cold temperature bleaching stating the 2010 cold event
just “resulted in some coral death.” However NOAA’s statement stands in
stark contrast to coral experts who reported the January 2010 cold snap was the worst coral bleaching and mortality event on record for
Florida’s Reef Tract. They reported, “the mean percent coral mortality
recorded for all species and subregions was 11.5% in the 2010 winter,
compared to 0.5% recorded in the previous five summers, including years
like 2005 where warm-water bleaching was prevalent.” Globally there has
been an increase in observed cold bleaching events and 2010 was
Florida’s first cold bleaching since the 1970s. Globally there have been several more reports of cold induced bleaching and then recovery as the waters warmed.
There
is a perception that bleaching suddenly became more common only since
the 1980s, leading some to speculate bleaching is due to rising CO2 and
global warming. However, whether warming since the Little Ice Age is
natural or anthropogenic, warming does not explain the increased
observations of cold bleaching. More frequent observations of bleaching
events may be partially due to the advent of remote sensing satellites
that have allowed greater global coverage only since the 1980s.
Furthermore determination of bleaching severity and mortality requires
teams of divers to ground truth satellite data and fine-tune percentages
of affected reefs. But SCUBA diving only became possible in the decades
after Jacques Cousteau invented the Aqualung in the 1940s. Although
natural rates of warming during the 30s and 40s were similar to today,
coral reef studies were also hampered by the unsafe battleground between
Japan and the Allies. War-time efforts such as the Battle of the Coral
Sea, and fights to control the islands of Peleliu, Midway, Iwo Jima, the
Philippines, or subsequent nuclear testing on the Bikini Atoll. The
resulting reef devastation likely obscured any natural bleaching events.
We
now know bleaching regularly happens due to seasonal fluctuations
between high solar irradiance and warm temperatures of summer versus
lower irradiance and cooler temperatures in winter. High irradiance can
damage the corals’ symbiotic algae when photosynthesis runs too rapidly,
while low irradiance detrimentally reduces photosynthetic output. Thus
coral undergo natural adjustments to seasonal changes by expelling a portion of their symbiotic algae in summer.
This leads to temporary or partial bleaching. Low light and colder
temperatures slow photosynthesis, so coral increase their symbiont
density in winter.
Similarly
in response to changes in sunlight, the same species will alter their
symbiotic partnerships as irradiance declines at increasing depths or
when and where water turbidity alters irradiance. Bleaching is often
temporary and mild as coral shuffle and switch their symbiotic algae in
order to adapt, but sustained extremes, warm or cold, can prolong
bleaching and starve the coral. Whether coral die or not depends on how
quickly new symbionts are acquired relative to how much energy the coral
has stored, or coral’s ability to feed on plankton as an alternative
energy source.
All
recent global bleaching events have been driven by El Nino events. The
1998 El Nino caused widespread mortality, an estimated 16% globally.
Observed bleaching in response to warm tropical waters invading cooler
regions aroused fears that climate change had contributed to this
“unprecedented” event. However researchers have noted the relationship
between warmer ocean temperatures and “bleaching has been equivocal and sometimes negative
when the coolest regions were not in the analyses.” In other words
coral living in the warmest waters were well acclimated to the warmest
waters redistributed by an El Nino. Furthermore mortality did not always
occur during periods with the warmest temperatures, but during the
winter or ensuing cold La Nina conditions. Such observations suggest the
rapid swings between anomalously warm El Nino and anomalously cold La
Nina conditions are the most stressful.
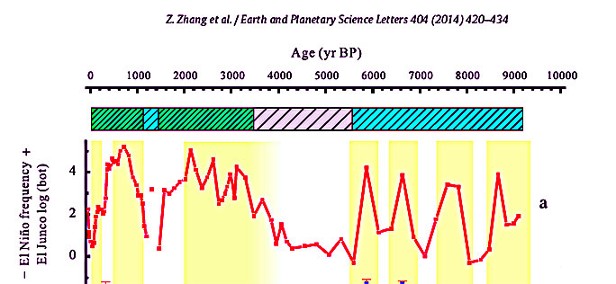
Stressful
rapid temperature variations due to El Nino events have occurred
throughout the past 10,000 years. As illustrated below from Zhang 2014,
the frequency of El Ninos during the past century has been neither
extremely high, nor extremely low. Most living coral species have
survived over a million years of climate change and have endured the
extreme El Nino frequencies of the past 3000 years including the Little
Ice Age. El Nino events are a function of natural ocean variability and
there is no consensus regards any effect from rising CO2 on El Nino
frequency or intensity. To survive extremes from past natural
variability, coral species had to be extremely resilient in ways that
are just now being understood.
2. Bleaching Causes the Least Mortality
Most
extreme bleaching events are associated with El Ninos, but the high
mortality rates are not just a function of higher temperatures. Due to
associated flooding and high rainfall, the resulting change in salinity
disrupts coral osmosis, which can result in coral death. Furthermore
tropical storms and heavy wave action are a major cause of lost coral
reefs, but storms also bring heavy rains that also induce bleaching.
Although some try to link storm-related mortality to climate change,
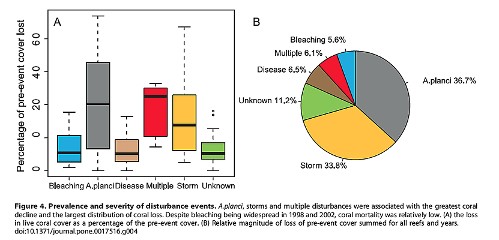
there is no evidence of an increasing trend in tropical storms. As illustrated by the pie graph from Osborne 2011,
in the Great Barrier Reef the explosion of the coral-eating Crown of
Thorns starfish (A. planci) and tropical storms contributed to the
greatest loss of coral colonies, 70.5%. Bleaching is a very minor
contributor to coral mortality, just 5.6%, and that bleaching can be
induced by warm or cold temperatures, heavy rains and floods or high
irradiance from anomalously clear skies.
Due
to coral’s symbiotic efficiency and recycling of nutrients, corals
dominate in nutrient-limited tropical waters. Normally those low
nutrient conditions also prevent predators like the Crown of Thorns
starfish (COTS) from rapidly reproducing because their plankton-feeding
larvae typically starve. But increased inflow of nutrients due to
landscape changes, agriculture run-off and sewage, has increased
plankton blooms and thus the survivorship of COTS’ larvae. The ensuing
population explosions of coral eating adults have decimated many reefs.
COTS does not exist in the Caribbean. Instead coral there are battling
bacterial diseases like white-band that can be spread by coral-eating
snails. Humans have indeed tipped the balance in favor of COTS and in
addition to destructive over fishing with dynamite and cyanide, those
causes of coral death are the only factors we can remedy.
To
understand coral resilience in the face of the variety of onslaughts,
coral reefs must be seen as dynamic systems that oscillate over decadal
periods, as well as centuries and millennia. Snapshots focused only on a
few years when coral reefs decline misrepresents coral resilience and
promotes false gloom and doom, as well as useless management plans. A
long-term study of coral ecosystems of an island in French Polynesia
demonstrates corals’ dynamics response to 32-years of storms, Crown of
Thorns starfish and bleaching. Coral mortality is often measured as a
function of the change in “coral cover”, and 45 to 50% of the healthy
reef system around the island of Tiahura was covered with coral.
As illustrated below in Figure 1 from Lamy 2016,
an outbreak of COTS removed 80% of the live coral cover between 1979
and 1982, reducing total coral cover to 10% of the reef. However by 1991
the coral had fully recovered. As designated by the small gray arrows
at the top, three bleaching events occurred during that recovery period.
Later destruction from a 1991 cyclone again reduced coral cover but
again coral recovered reaching its greatest coverage of 50% by the year
2000. And again during that recovery there were 3 more bleaching events.
Since 2006 the coral suffered their greatest loss due to another
outbreak of COTS, quickly followed by another cyclone. High mortality
promoted high seaweed cover (dotted green line) that has inhibited coral
recovery. Over that time, coral bleaching was associated with periods
of recovery, suggesting little if any detrimental effects. As will
become clear shortly, one also could reasonably argue those bleaching
events were beneficial.

3. Rapid Coral Recovery:
Tiahura’s
coral recovery periods typically required 7 to ten years, and appeared
to be unaffected by the 1998 El Nino. Several other studies have
reported similar recovery periods, but some locations required 10 to 20
years to fully recover. In Australia’s Great Barrier Reef (GBF), the
1998 El Nino induced above average sea surface temperatures and salinity
changes for 2 months triggering massive coral losses in the reef’s
upper 20 meters. At (GBR)Northwest
Australia's Scott Reef, the upper 3 meters lost 80 to 90% of its living
coral and the disappearance of half of the coral genera. Yet researchers
observed, “within 12 years coral cover, recruitment, generic diversity,
and community structure were again similar to the pre-bleaching
years.” A similar long-term study
in the Maldives observed a dramatic loss of coral during the 1998 El
Nino but by 2013 the reefs also had returned to “pre-bleaching values”.
Although a reef’s recovery sometime requires re-colonization by larvae
from other reefs, a process known as re-sheeting or Phoenix effect
can facilitate a reef’s speedy recovery. Often a small percentage of
living “cryptic” polyps with a more resilient symbiotic partnership were
embedded within a “dead” colony and survive extreme bleaching. They
then multiply and rapidly “re-sheet” the colony’s skeletal remains.
In addition to rapid recovery of coral cover, researchers are finding bleached reefs have been increasingly less susceptible to subsequent bleaching. For example studies
in Indonesian waters determined that two coral species, highly
susceptible to bleaching, had experienced 94% and 87% colony deaths
during the 1998 El Nino. Yet those same species were among the least
susceptible to bleaching in the 2010 El Nino, with only 5% and 12%
colony deaths despite a similar increase in water temperatures.
Similarly, changes in resilience were observed in response to cold water
bleaching in the Gulf of California. Increased resilience in response to a variety of bleaching events prompted the Adaptive Bleaching Hypothesis
first proposed in 1993. The hypothesis suggests that although bleaching
events are a response to stress, it creates the potential for coral to
acquire totally new and different symbionts that are better suited to
those stressful conditions. Contrary to Hoegh-Guldberg’s
claim that coral reef systems will “experience near annual bleaching
events that exceed the extent of the 1998 bleaching event by the year
2040”, scientists are increasingly observing the exact opposite. After
reefs recover from severe bleaching, colonies have evolved enhanced
resilience to future bleaching.
4. Coral Symbiosis, Symbiont Shuffling and Rapid Adaptation

A
single coral colony is comprised of 100s to millions of individual
“polyps” (seen above). Each polyp can be visualized as an upside down
jellyfish (coral’s close cousins) with their backs cemented to a surface
and tentacles extended outward to capture passing food particles, live
prey, or new symbionts. However because coral live in nutrient depleted
environments, in addition to filter feeding, polyps harbor single-celled
photosynthesizing symbionts inside their cells. Those symbionts (aka
zooxanthellae) typically provide ~90% of the coral’s energy needs. Just
40 years ago it was believed all corals were host to just one
photosynthesizing symbiont, a single species from the dinoflagellate
genus Symbiodinium. But thanks to technological advances in genetic
sequencing, we now know a coral species can harbor several potential
species or types of Symbiodinium algae, each capable of responding
optimally to a different set of environmental conditions and coral
physiology. As predicted by the adaptive bleaching hypothesis, improved
genetic techniques have revealed a wondrously diverse community of symbionts
that coral can choose from. Coral can no longer be viewed as organisms
that only adapt slowly over evolutionary millennia via genetic mutation
and natural selection. Coral must be seen as an “eco-species” (aka
holobiont) that emerges from the synergy of the coral and its varied
symbionts. And we now know those emergent eco-species can rapidly
evolve with changing climates by shuffling and shifting those symbionts.
A
single colony’s polyps are typically all clones resulting from asexual
reproduction and on their own offer the colony scant genetic
versatility. However within a colony, a wide variety of symbionts can be
harbored within a small percentage of polyps, although one symbiont
type typically dominates. That small percentage of “cryptic” polyps
often survive severe bleaching episodes and then multiply rapidly over
the skeletal remains in a process known as the Phoenix effect. Just one
square centimeter of coral tissue typically harbors a million individual
symbionts and on average those symbionts can double every 7 days. Thus
after severe colony bleaching, a more resilient colony can arise in just
a few years with better-adapted symbionts now dominating. Likewise
symbiont variability within a reef results in some colonies bleaching
while adjacent colonies of the same species do not. And similarly a
varied symbiont and coral community allows neighboring reefs to adapt to
their unique regional climates.

Variations
in coral reproduction can conserve an “ecospecies” or rapidly promote
greater ecospecies diversity. Twenty-five percent of the coral species
produce larvae inoculated directly from their parent’s symbionts.
However 75% of the species produce larvae that initially lack a
symbiont. Only after coral larvae settle on a surface, do those larvae
engulf one or more different types of free-living Symbiodinium, drawing
them inside their cells. As the larvae develop into mature polyps, coral
typically keep the symbiont types best suited to the local microclimate
and expel the others. In this manner completely new eco-species emerge.
Furthermore as conditions change, all species can shuffle their symbionts
as polyps will expel their current residents and acquire a different
type that had been harbored by a neighboring polyp. A colony can also shift its symbiont population
by acquiring new types not yet hosted by the colony but are present in
the reef. Due to improving genetic techniques, previously undetected types of symbionts
with greater thermal tolerance are now being detected after bleaching
events. Thus a combination of symbiont shuffling and shifting is the key
to corals’ rapid adaptation. Although bleaching can result in coral
death due to starvation when new symbionts are not acquired quickly
enough, surviving polyps with their altered symbiont community have the
potential to re-direct the reef on a trajectory that is better suited to
the new environment. Or if conditions return to those prior to an
extreme event, coral can re-acquire their old symbiont types.
Scientists have found
that coral colonies nearer the surface often harbor a different type of
symbiont than colonies living just a few meters deeper. The symbionts
residing closer to the surface may be better adapted to high irradiance
by making proteins that protect against too much ultra violet light or
by modifying their photosystem. Conversely symbionts living at greater
depths may photosynthesize more efficiently under low light conditions
but are more susceptible to UV damage. Transplant experiments
revealed that when coral colonies growing at greater depths were
relocated closer to the surface, the polyps expelled their symbionts
resulting in temporary bleaching. Bleaching allowed polyps to acquire
new symbionts better adapted to higher irradiance. However colonies
adapted to high-light surface conditions, photosynthesized much more
slowly when transplanted to lower depths. Bleaching never happened and
the coral died. Although experiments can force bleaching by raising
temperatures, other controlled laboratory experiments
found that in the absence of stress from high solar irradiance,
anomalous temperatures 4 degrees above average still did not induce
bleaching.
According
to the adaptive bleaching hypothesis we can infer that bleaching events
are not simply the result of recent global warming. Bleaching should
have been ongoing for millions of years, as background temperatures have
risen and fallen. Thus we would expect that as the Little Ice Age ended
and temperatures naturally rose, there should be observations of
bleaching in the early 1900s. And indeed there are albeit limited. For
example bleaching was reported in Florida on hot days in the early
1900s. But more telling, enough warm weather bleaching had been observed
in the early 20th century that the Great Barrier Reef expedition of 1928-29 focused on warm weather coral bleaching when oceans were cooler than today and long before any possible CO2 warming effect.
Coral Response to Climate Change
Since his first Greenpeace-funded 1999 study,
Hoegh-Guldberg has promoted catastrophic climate change as the biggest
threat to coral reefs. His papers are frequently cited as evidence of
climate related coral demise by some researchers and hyped by media
outlets that boost readership by promoting climate catastrophes. The
bases for his claims relied on 3 simplistic assumptions that a)
bleaching is evidence that coral have reached their limit of maximum
thermal tolerance, b) bleaching will increase due to global warming, and
c) coral cannot adapt quickly enough to temperatures projected by
climate models.
In
1999 Hoegh-Guldberg argued “thermal tolerances of reef-building corals
will be exceeded within the next few decades” and coral reefs "could be eliminated from most areas by 2100"
due to climate change. In his 2014 paper he continued to dismiss the
emerging science supporting the adaptive bleaching hypothesis,
belittling it as a “persistent mirage”.
His catastrophic claims also intensified, suggesting “as much as 95%
[of the world’s coral] may be in danger of being lost by mid-century.”
To support his extirpation claim he cited two of his own previously
published papers. Hoegh-Guldberg’s history of exaggeration and circular
reasoning has led other coral experts to accuse him of “popularizing worst case scenarios”, while others have accused him
of persistently misunderstanding and misrepresenting the adaptive
bleaching hypothesis. Furthermore other researchers have pointed out the
pitfalls and weaknesses in framing threats to coral based on a
simplistic temperature threshold. They argue,
“A view of coral reef ecosystems that emphasizes regional and
historical variability and acclimation/adaptation to various
environments is likely to be more accurate than one that sees them as
characterized by stable and benign temperature regimes close to their
upper thresholds.”
As one of many examples of his deceptive misstatements, in his 2014 paper
Hoegh-Guldberg wrote, “there is little evidence that acclimatisation
has resulted in a shift or extension of the upper thermal tolerance of
reef-building corals [42].” His citation simply referenced a paper he
had co-authored. But in that paper he admitted never identifying the
symbionts or trying to detect any symbiont shuffling or shifting.
Furthermore his methodology removed coral from their potential symbiont
community during experimental heat stress treatments, minimizing any
possibility for the coral to switch symbionts. But it is symbiont
shifting that allows coral to shift their upper thermal tolerance
levels. Hoegh-Guldberg’s basis for claiming “little evidence” was
totally irrelevant, if not dishonest.
In
contrast, improved genetic sequencing is increasingly providing
evidence that in response to warm water bleaching events coral begin
acquiring new heat resistant symbionts. The results below from Boulotte 2016
show that over the course of 2 years, colonies radically altered their
symbionts. The pie charts represent the changing percentage of dominant
symbiont types due to shuffling in a single reef species. The bar graphs
list just the rarer symbionts and stars identify types not previously
detected suggesting an ongoing shift. Symbionts “types” are
characterized first by their genetic lineages known as clades. When the
adaptive bleaching hypothesis was first proposed, only 4 clades were
known. Now at least nine have been identified. The most heat resistant
symbionts belong to clade D, but other heat resistant types have evolved
within other clades. Many earlier acclimation studies simply identified
a symbiont’s clade. But we now know each clade can harbor hundreds of
types (potential species) and improved detection of those species is
uncovering more shifting. The most heat resistant species
identified to date belonged to clade C. As seen here, different
types/species are identified as D_I:6 or D1.12. As illustrated below
after 2 bleaching episodes, a new symbiont species from clade C began to
dominate and previously undetected clade D symbionts began to appear
more frequently in just 2 years.

Nevertheless
Hoegh-Guldberg 2014 continues to dismiss coral’s ability to rapidly
adapt arguing, “current rates of change are unprecedented in the past 65
Ma [million years] if not 300 Ma.” But such exaggeration is pure
nonsense. Ocean temperatures were warmer just 1000 years ago, and
paleo-studies of temperatures in the Great Barrier Reef suggest local
reef temperatures were higher between 1720 and 1820 as illustrated below
from Hendy 2003.
(Their luminescence index measures changes in salinity associated with
monsoons). Perhaps CO2 concentrations are higher now than over the last
300 Ma. But given the extreme warmth just 65 million years ago, that is
evidence that our climate is not very sensitive to CO2 concentrations,
as realized by more researchers. In contrast to IPCC models that predict more warming that Hoegh-Guldberg ties to coral demise, climate experts note the Holocene temperature conundrum.
While CO2 driven models simulate 6000 years of warming due to rising
CO2, all the proxies indicate a cooling trend interrupted only by
warming spikes.

Although
coral genomes may evolve slowly, their symbionts have extremely fast
generation times, averaging every 7 days. Furthermore the symbiont
community consists of hundreds of symbionts that have already adapted to
a wide variety of temperature, irradiance and salinity variables within
different microclimates over the past million years. Symbiont shuffling
and shifting is an evolutionary masterpiece that circumvents plodding
evolutionary mechanisms of most organisms with long generation times and
enables immediate adaptation. To counter the emerging science,
Hoegh-Guldberg can only invoke silly semantics to argue symbiont
shifting is not “true adaptation”. But again his arguments evoke
criticism from his colleagues who wrote, “flexibility in coral–algal symbiosis is likely to be a principal factor underlying the evolutionary success of these organisms”.
But
Hoegh-Guldberg seems less interested in embracing the emerging science
of coral resilience, in order to cling to his belief in catastrophic
climate change.

No comments:
Post a Comment